The use of sound wave to targeting Cancer
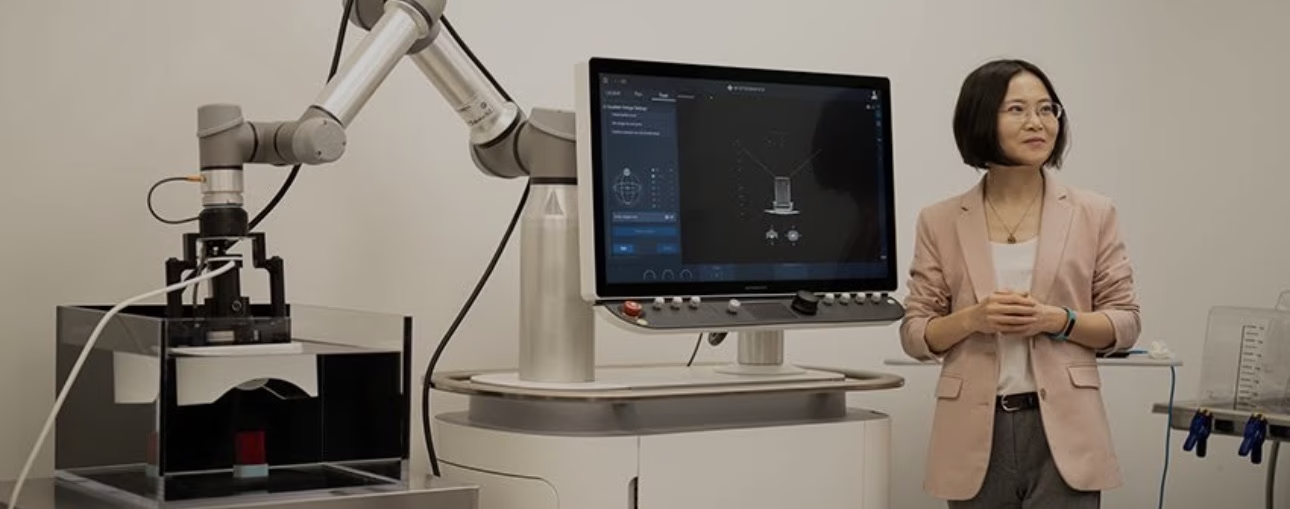
Ultrasound has long been used for helping doctors see inside the body, but focused high frequency sound waves are offering new ways of targeting cancer.
If Zhen Xu hadn’t annoyed her lab mates, she might never have discovered a groundbreaking treatment for liver cancer.
As a PhD student in biomedical engineering at the University of Michigan in the US during the early 2000s, Xu was trying to find a way for doctors to destroy and remove diseased tissue without the need for invasive surgery. She’d landed on the idea of using high-frequency sound waves – ultrasound – to mechanically break up tissue and was testing her theory on pig hearts.
Ultrasound isn’t supposed to be audible to human ears, but Xu was using such a powerful amplifier in her experiments that other researchers she shared the laboratory with began to complain about noise. “Nothing had worked anyway,” she says. So she decided to humour her colleagues by increasing the rate of ultrasound pulses, which would bring the sound level outside the range of human hearing.
To her shock, increasing the number of pulses per second was not only less disruptive to those around her, but also more effective on living tissue than the approach she’d tried previously. As she watched, a hole appeared in the pig heart tissue within a minute of ultrasound application. “I thought I was dreaming,” says Xu, who is today a professor of biomedical engineering at the University of Michigan.
Decades later, Xu’s serendipitous discovery, known as histotripsy, is one of several approaches using ultrasound that are ushering in a new era of advanced cancer treatment, offering doctors non-invasive methods to rid patients of cancerous tumours using sound rather than surgery.

Histotripsy was approved by the US Food and Drug Administration for the treatment of liver tumours in October 2023. The following year, a small study funded by HistoSonics, the company formed to commercialise Xu’s technology, found that the approach achieved technical success against 95% of liver tumours. While side effects ranging from abdominal pain to internal bleeding are possible, research suggests complications are rare and the method is generally safe.
In June, the UK became the first European country to approve histotripsy. The treatment was made available on the NHS under the pilot phase of its Innovative Devices Access Pathway for unmet clinical needs.
“People think of ultrasound as imaging,” says Julie Earl, a principal investigator in the biomarkers and personalised approach to cancer group at Spain’s Ramón y Cajal Institute for Health Research who has studied the technology. But a growing body of research, she says, suggests it can also destroy tumors, subdue metastatic disease (cancers that have spread to other parts of the body) and boost the efficacy of other cancer treatments – all without putting a patient under the knife.
How ultrasound works
For many people, the word “ultrasound” triggers instant associations with sonograms during pregnancy. To create a medical image like a sonogram, a handheld transducer sends high-frequency sound waves into the body, where they ricochet off tissues inside. A sensor in the device picks up the waves that bounce back, converting their activity into electrical signals, which are then used to create an image of what’s going on beneath the skin.
In cancer treatment, ultrasound waves are concentrated onto a small area of a tumour to destroy it.
These pulses create tiny microbubbles that expand and then collapse in microseconds, breaking apart the tumour tissue as they do
For treatment of liver cancer, histotripsy devices channel ultrasound waves into a focal zone of about two by four millimetres – “basically, the tip of your colouring pen”, Xu says. Then, a robotic arm guides the transducer over the tumour to target the correct area.
The ultrasound is delivered in quick bursts. These pulses create tiny “microbubbles” that expand and then collapse in microseconds, breaking apart the tumour tissue as they do. The patient’s immune system is then able to clean up the remains.
The whole thing is fast, non-toxic and non-invasive, usually allowing patients to go home the same day, Xu says. While exact treatment time varies, most procedures are over in one to three hours, according to HistoSonics. Tumours are often destroyed in a single session, although patients with multiple or larger lesions may need multiple rounds.
While its benefits are promising, there are unanswered questions about histotripsy. There’s not yet robust long-term data about cancer recurrence after treatment. Some researchers have raised concerns about histotripsy potentially seeding new cancer growths as tumours are broken up inside the body, meaning they can be transported to other areas. That fear, however, hasn’t borne out in animal studies so far.

Histotripsy may also not work against all cancers, research suggests. Bone can block ultrasound from reaching its intended source, ruling out tumours in certain locations. And using histotripsy in gaseous organs, like the lungs, could be dangerous, potentially causing damage to nearby healthy tissues. But HistoSonics is currently studying histotripsy as a potential treatment for tumours of the kidney and pancreas.
Cooking cancers with ultrasound
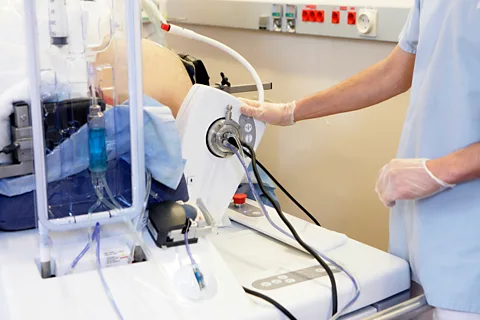
Histotripsy is not the first use of ultrasound in cancer care. High-intensity Focused Ultrasound (HIFU), an older and more established technology, can also be used to attack tumours. A focused blast of ultrasound is applied to a tumour to generate heat that essentially “cooks” the tissue, says Richard Price, who co-directs the Focused Ultrasound Cancer Immunotherapy Center at the University of Virginia in the US.
“If you take a magnifying glass and you hold it outside on a sunny day over a dry leaf, you could actually start the leaf on fire,” Price says. HIFU essentially does the same thing to cancer tissue, only using sound energy.
In oncology, HIFU is perhaps best known as a non-invasive way to treat prostate cancer, an application for which it seems to be roughly as effective as surgery, according to a 2025 study. Patients may experience some pain and urinary side effects when they wake up, but recovery is generally faster than after intensive therapies like surgery.
Both histotripsy and HIFU therapy are typically performed under general anaesthesia so patients don’t move during treatment, minimising the possibility of accidental damage to nearby organs or tissues. But histotripy does not generate the heat produced by HIFU, which can damage nearby healthy tissue.
Not all cancers can be treated with HIFU, however – again, bone or gas can block ultrasound from reaching tumours. It’s not generally an optionfor patients whose prostate cancer has spread throughout the body. Still, researchers in numerous countries are studying it in the hope of targeting other cancers, including some forms of breast cancer.
Ultrasound plus other drugs
The power of ultrasound could also be supercharged by combining it with other existing forms of cancer treatment, researchers say.
Recent research suggests, for example, that injecting microbubbles into the bloodstream and stimulating them with ultrasound can temporarily open the blood-brain barrier. This barrier usually prevents toxins in the bloodstream from entering and damaging the brain. But purposely opening it during cancer treatment could allow drugs to reach the tumours they’re meant to attack.
“The non-invasive part is awesome, but the [drug-delivery component] is really unmatched anywhere,” Price says.
Deepa Sharma, a research scientist at Sunnybrook Health Sciences Centre in Ontario, Canada, says those benefits don’t stop at brain cancer. She has studied the combination of ultrasound and microbubbles across cancer types, finding that it can broadly improve drug delivery.
Sharma’s research also suggests that ultrasound-enhanced microbubbles can boost the effects of radiation by damaging the vasculature of tumours, leading to greater cell death. Those findings suggest that doctors can use smaller amounts of toxic cancer treatments, like chemotherapy and radiation, if they’re paired with ultrasound and microbubbles, she says.
As focused ultrasound heats and damages tumours, it seems to make these tissues more visible to the immune system
“Radiation therapy does cure cancer, but it does also cause a lot of long-term side effects,” Sharma says. If its effects can be enhanced thanks to ultrasound-stimulated microbubbles, she says, doctors could theoretically use lower doses to achieve the same treatment effects with fewer devastating side effects.
Ultrasound also seems to be a good match for immunotherapy, a treatment approach focused on spurring the immune system to fight off cancerous cells that may be dodging or hiding from the body’s natural defences.
As focused ultrasound heats and damages tumours, it seems to make these tissues more visible to the immune system – and thus more vulnerable to its defences, says Price, whose research centre is focused on using ultrasound along with immunotherapy.
A direction for future research, Price says, is determining whether that pairing can work against advanced-stage cancer. Metastatic cancer is much harder to treat than localised disease – when cancer spreads throughout the body, it’s no longer enough to remove a single tumour. The holy grail would be that clinicians could someday use ultrasound to coax one tumour out of hiding by breaking it apart, allowing the immune system to pick up on its characteristics and launch a system-wide attack against cancerous cells elsewhere in the body, Price says. This remains to be tested in any kind of trials, but theoretically, doctors could “treat 10, 15, 20 tumours just by treating one tumour”, Price says.

That said, trials on ultrasound and immunotherapy are still in relatively early stages, Price cautions. That means far more research is required to know if, when or how this combined approach could change patient care.
But ultrasound approaches already in use are ushering in a new era in oncology – one that aims to replace, or at least improve, effective-yet-devastating therapies like surgery, chemotherapy and radiation. “Cancer is awful,” Xu says. “What’s making it even worse is cancer treatment.”
Ultrasound isn’t a “magic cure” for cancer, Xu says. Like any medical treatment, it has downsides and shortcomings. But just as she was able to spare her lab mates from annoying noise decades ago, Xu hopes her discovery – and those of other scientists – will help patients avoid unnecessary suffering for years to come.

